Motivation
What do we measure?
The atom consists of a nucleus, containing protons and neutrons, surrounded by electrons.
The properties of the nucleus (shape, spin, moments) affect the energy levels of the surrounding electrons.
By measuring the energy levels of the electrons, we can extract information about the nucleus.
If the nucleus was the size of a pea, the electrons would be orbiting 1 km away. You wouldn’t see it and yet we can measure its size with better than 1% precision!
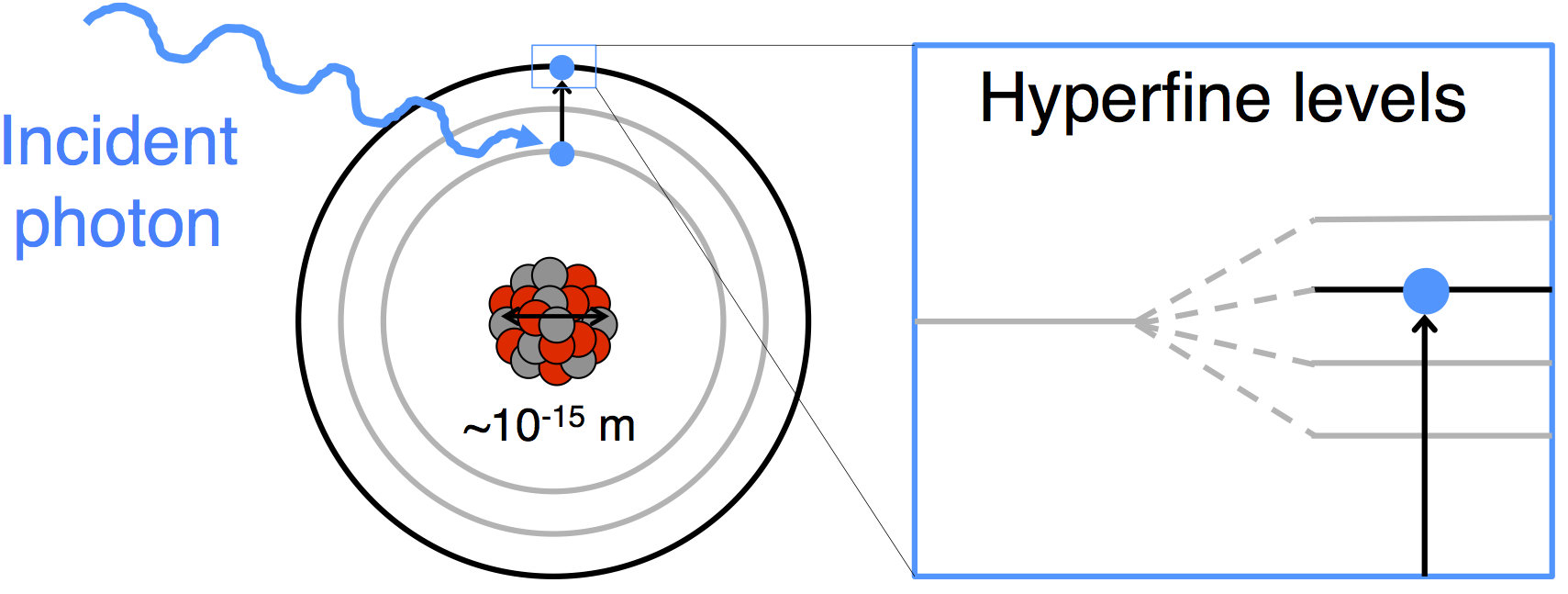

How do we measure it?
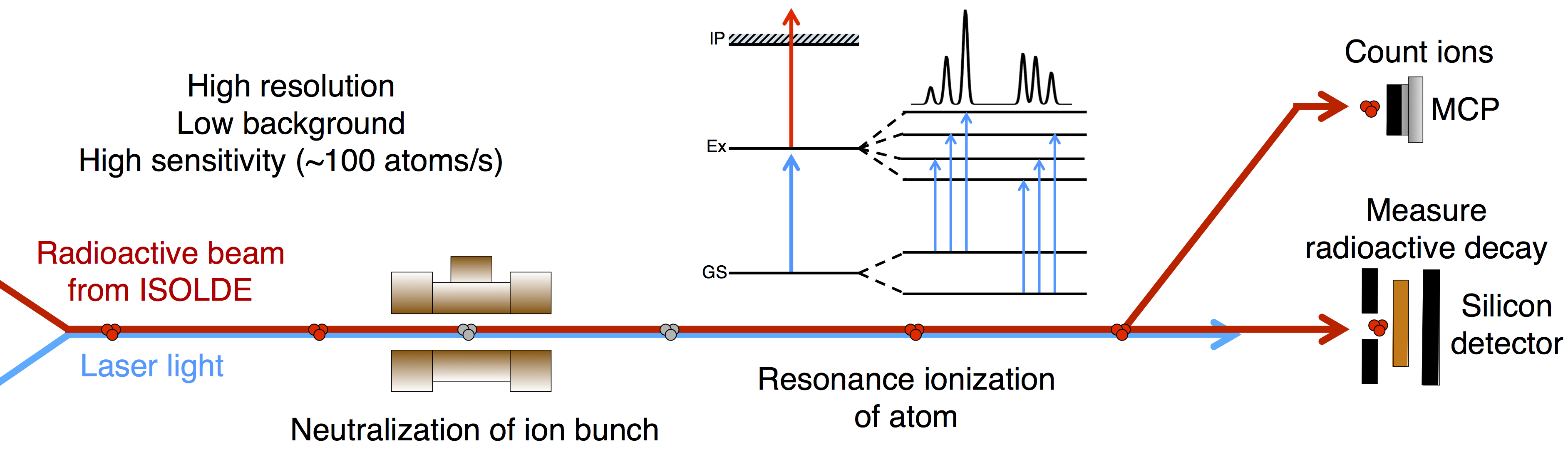
What does that tell us?
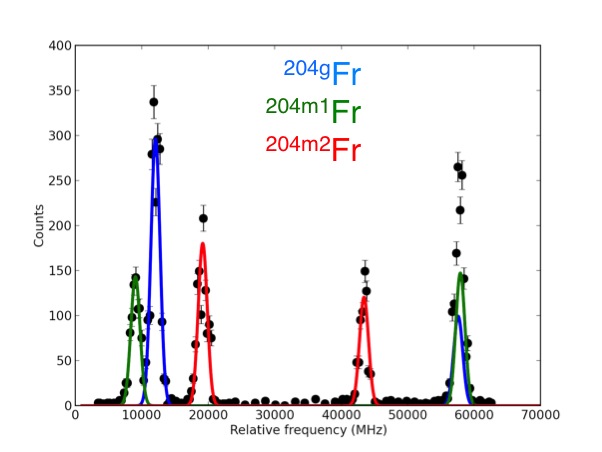
Probing the energy levels of the electrons allows fundamental nuclear properties to be measured, without relying on nuclear theory.
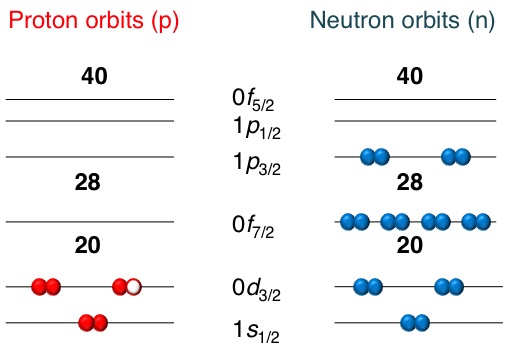
Measuring the magnetic dipole moment provides information on the configuration of the protons and neutrons in the nucleus.
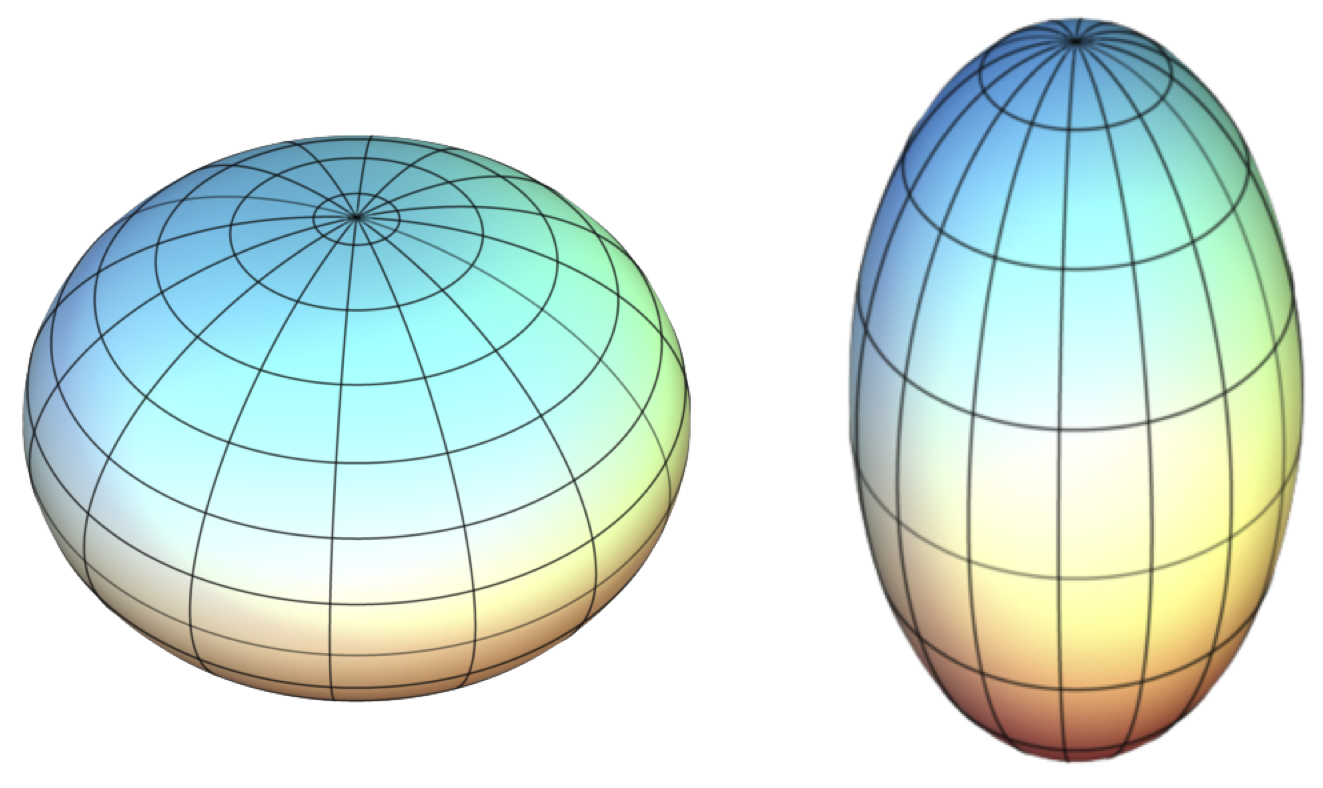
Relative charge radii tell us how the shape of the nucleus changes with the addition and subtraction of neutrons.
Nuclear spin tells us the angular momentum of the nucleus.
Electric quadrupole moment provides information on the static deformation of the nucleus.